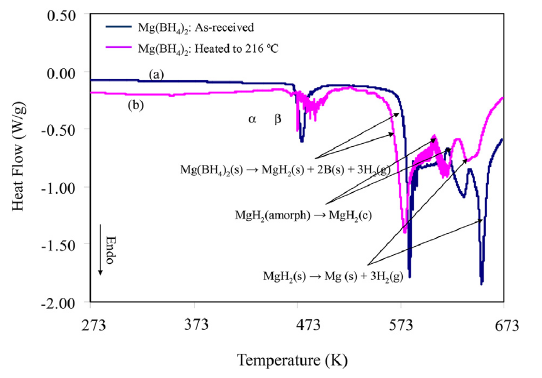
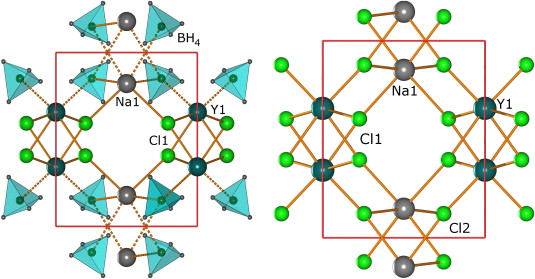
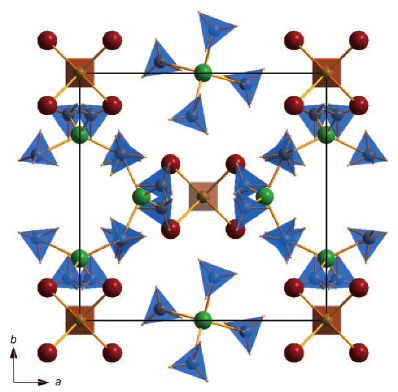
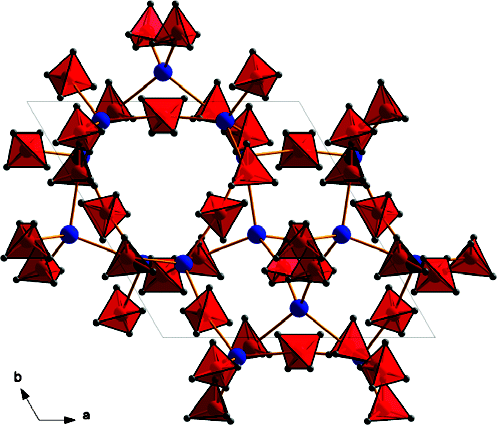
A new potassium scandium borohydride, KSc(BH4)4, is presented and characterized by a combination of in situ synchrotron radiation powder X-ray diffraction, thermal analysis, and vibrational and NMR spectroscopy. The title compound, KSc(BH4)4, forms at ambient conditions in ball milled mixtures of potassium borohydride and ScCl3 together with a new ternary chloride K3ScCl6, which is also structurally characterized. This indicates that the formation of KSc(BH4)4 differs from a simple metathesis reaction, and the highest scandium borohydride yield (~31 mol %) can be obtained with a reactant ratio KBH4:ScCl3 of 2:1. KSc(BH4)4 crystallizes in the orthorhombic crystal system, a = 11.856(5), b = 7.800(3), c = 10.126(6) Å, V = 936.4(8) Å3 at RT, with the space group symmetry Pnma. KSc(BH4)4 has a BaSO4 type structure where the BH4 tetrahedra take the oxygen positions. Regarding the packing of cations, K+, and complex anions, [Sc(BH4)4]−, the structure of KSc(BH4)4 can be seen as a distorted variant of orthorhombic neptunium, Np, metal. Thermal expansion of KSc(BH4)4 in the temperature range RT to 405 K is anisotropic, and the lattice parameter b shows strong nonlinearity upon approaching the melting temperature. The vibrational and NMR spectra are consistent with the structural model, and previous investigations of the related compounds ASc(BH4)4 with A = Li, Na. KSc(BH4)4 is stable from RT up to ~405 K, where the compound melts and then releases hydrogen in two rapid steps approximately at 460−500 K and 510−590 K. The hydrogen release involves the formation of KBH4, which reacts with K3ScCl6 and forms a solid solution, K(BH4)1−xClx. The ternary potassium scandium chloride K3ScCl6 observed in all samples has a monoclinic structure at room temperature, P21/a, a = 12.729(3), b = 7.367(2), c = 12.825(3) Å, β = 109.22(2)°, V = 1135.6(4) Å3, which is isostructural to K3MoCl6. The monoclinic polymorph transforms to cubic at 635 K, a = 10.694 Å (based on diffraction data measured at 769 K), which is isostructural to the high temperature phase of K3YCl6. |